Nice Info About How To Remember Cathode And Anode

Catodo é Positivo Ou Negativo
Cracking the Code
1. Unraveling the Mystery of Electrodes
Alright, lets be honest. How many times have you stared blankly at a circuit diagram, completely blanking on which electrode is the cathode and which is the anode? It happens to the best of us! These two little terms can be surprisingly tricky to keep straight. But don't worry; this isn't some kind of electrical engineering hazing ritual. We're going to decode this once and for all, using clever tricks and memorable mnemonics.
Think of it this way: electricity, much like a good story, needs a beginning and an end. The cathode and anode are simply the two ends of that electrical story, the entry and exit points for the current's flow. Understanding their roles is crucial for understanding how batteries, circuits, and all sorts of cool gadgets actually work.
So, buckle up as we journey into the electrifying world of charged particles. We'll explore the differences between these electrodes, the roles they play, and most importantly, how to nail down the distinction in your memory forever. No more electrode-related brain farts, I promise!
Because let's face it, confusing these two can lead to some pretty epic face-palm moments in your electronics projects. I mean, who wants to accidentally reverse polarity and fry their Arduino? Not me! Let's get this sorted.

Best Trick To Memorize Charge On Cathode And Anode YouTube
Negative Nelly and Positive Pete
2. Using Mnemonics to Master Electrode Identification
Okay, time for the secret weapon: mnemonics! These little memory tricks can be absolute lifesavers when you're faced with a potentially confusing situation. So, heres my personal favorite to keep these terms straight. Think of the anode as the "A-ttracting Negative charge". Remembering this simple phrase can act as a constant reminder that it attract the negative charge or negatively charged particles(anions).
And for the cathode, you can use the phrase "Cathode Catches Positive." This highlights the fact that a cathode will attract positive charge or positively charged particles(cations). If you can picture a little cartoon cat catching positive charges with its paws, you're golden! It's silly, sure, but silliness is the key to making it stick in your brain.
Another way to think about it, which I learned back in my early days of tinkering, is to associate "ANode" with "A Negative destination." This implies that the anode is the final resting spot for negative charge. The negative charge flows towards the anode. Then, by default, the cathode must be the source or the starting point for the positive charge. Or simply remember the Anode starts with A like attraction of negative charges.
The beauty of these mnemonics is that they provide a mental anchor. When you're facing that daunting circuit diagram, just conjure up the image of a cat catching positive ions or visualize the anode attracting negative charges. The fog of confusion will lift, and the answer will be clear.

Context is Key
3. How the Roles Reverse Based on the System
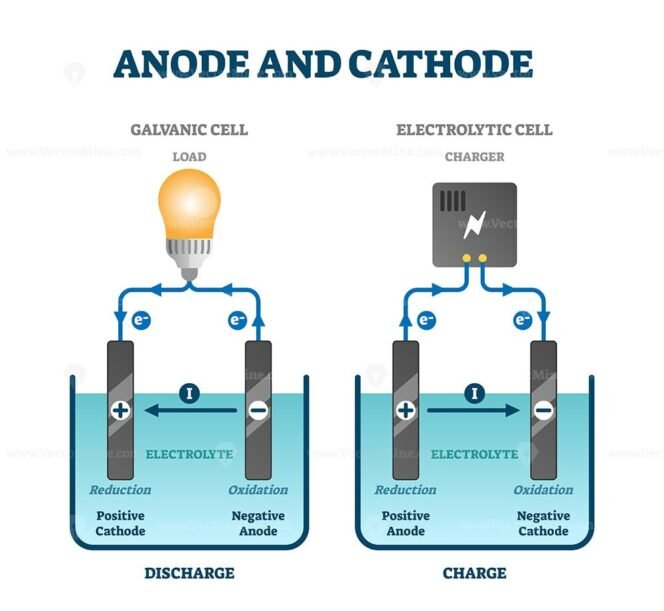
Now, here's a twist: the roles of the anode and cathode can actually switch depending on whether you're dealing with a battery (galvanic cell) or an electrolytic cell. Tricky, right? But stick with me, it is logical when you understand the underlying principles.
In a battery, the anode is the negative electrode, and it's where oxidation occurs (loss of electrons). The cathode is the positive electrode, and it's where reduction occurs (gain of electrons). Think of a battery as providing power, and electrons flow from the negative anode to the positive cathode to do work.
However, in electrolysis (like electroplating or separating water into hydrogen and oxygen), things flip. The anode becomes the positive electrode, and it's where oxidation still occurs. The cathode becomes the negative electrode, and it's where reduction still occurs. The difference is that you're forcing the reaction using an external power source.
To remember this, consider what you are doing: Is the cell producing electrical energy (battery) or consuming electrical energy (electrolysis)? If it's producing, the anode is negative. If it's consuming, the anode is positive. The key takeaway is that oxidation always happens at the anode, and reduction always happens at the cathode, regardless of the cell type.

The Lingo Lowdown
4. Understanding the Underlying Chemical Reactions
Let's dive into the slightly more technical side of things. The terms "oxidation" and "reduction" are absolutely crucial for understanding what's happening at the anode and cathode. These reactions are the heart and soul of electrochemical processes, and understanding them truly cements your grasp on electrode functionality.
Oxidation, as mentioned earlier, is the loss of electrons. Think of it as "oxidation is losing" or OIL (Oxidation Is Loss). When a substance oxidizes, it essentially gives up electrons. This process always happens at the anode, regardless of the type of electrochemical cell.
Reduction, on the other hand, is the gain of electrons. Remember it as "reduction is gaining" or RIG (Reduction Is Gain). When a substance reduces, it accepts electrons. And you guessed it, this process always happens at the cathode.
So, heres the golden rule: Anode = Oxidation; Cathode = Reduction. Remembering "An Ox and Red Cat" can also aid you in keeping this straight. It is a weird phrase, but odd phrases are easier to remember. Understanding this fundamental principle allows you to predict the direction of electron flow and the chemical changes happening in the cell.
Real-World Relevance
5. From Batteries to Electroplating
So, why bother learning all this stuff? Well, cathodes and anodes aren't just abstract concepts floating in the ether. They're everywhere! Understanding them unlocks a deeper appreciation for how many technologies actually work.
Batteries, of course, are the most obvious example. Every battery has a cathode and an anode, and the flow of electrons between them generates the electricity that powers our phones, laptops, and countless other devices. Understanding which terminal is which is essential for proper use and safety.
Electroplating is another important application. This process uses electrolysis to coat a metal object with a thin layer of another metal. The object to be plated acts as the cathode, while the metal being plated acts as the anode. Think about chrome plating on cars or gold plating on jewelry — it's all thanks to cathodes and anodes working their magic.
Even in biological systems, cathodes and anodes play a role. In nerve cells, for example, the movement of ions across the cell membrane creates electrical potentials, effectively creating tiny biological batteries. And in medical devices like pacemakers, electrodes are used to stimulate heart muscle contractions.
FAQ
6. Q
A: Use mnemonics! "Anode Attracts Negative" and "Cathode Catches Positive." Visualizing these phrases can make it much easier to remember which electrode attracts which type of charge. Also remember "An Ox and Red Cat".
7. Q
A: Not necessarily! In a battery (galvanic cell), the anode is negative. In an electrolytic cell, the anode is positive. The key is to remember that oxidation always occurs at the anode, and reduction always occurs at the cathode.
8. Q
A: It's crucial for understanding how circuits, batteries, and other electrochemical devices work. Incorrectly identifying them can lead to wiring errors, damaged equipment, or even safety hazards.